In order to dissolve a compound to make a solution of a specified concentration, you need to know its molecular weight. Most compounds are sold in a pure (non-salt) free form and it is clear what the molecular weight is: but a salt chemical compound is sold in a salt form, which means that you need to calculate the effective molecular weight that takes into account the compound to salt weight ratio. We therefore strongly prefer the salt information to be included in the structure diagram of the product, as in this example:
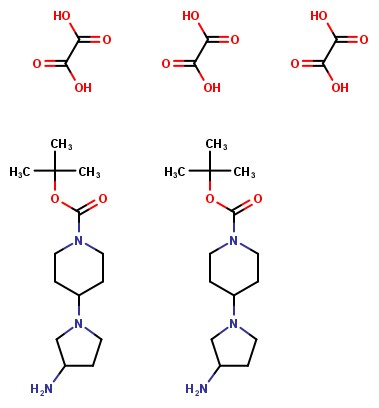
tert-butyl 4-(3-aminopyrrolidin-1-yl)piperidine-1-carboxylate has molecular formula C14H27N302 and molecular weight 269.39 g mol-1.
The effective molecular weight can then be normalized to one molecule of the compound and used to calculate the weight of compound needed for a particular concentration. For the example above dot-disconnected is formula C14H27N302.1:1.5C2H2O4 and effective molecular weight 404.44 g mol-1.
Suppliers don’t always include salt data in the chemical structure diagram of the compound. A common approach is to include a field called Salt or Salt Data where the salt forms identity and stoichiometric ratio can be provided.
There are over 1600 unique salt comments in the MolPort database. This means that a given salt may be described in text in multiple ways.
Here are some examples we see for representing the chloride salt of a compound:
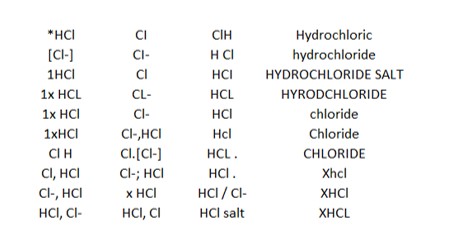
When stoichiometry and salt and solvate combinations are included, there are even more variations.
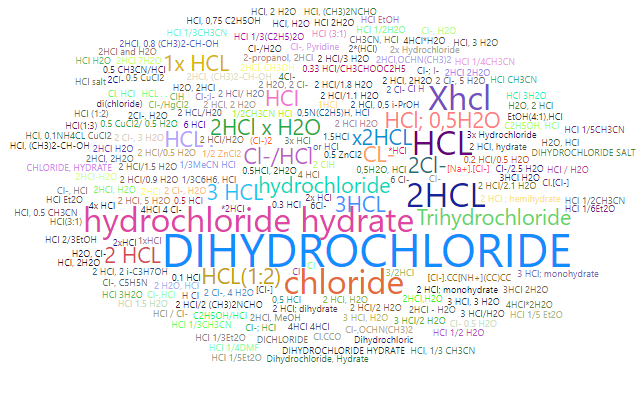
Here are the 100 most commonly encountered salt field comments:

While it is feasible to manually calculate the correct effective molecular weight for one or a few compounds, it is nevertheless inconvenient. For large library acquisitions, manual interpretation and calculation are simply not practical. At MolPort we have been working on a process that interprets supplier-provided salt data comments and adds this information to the chemical structure of a product.
Currently we are successfully processing approximately 99% of the salt comments. When we update supplier catalogs, this information is transformed and added to the chemical structure. The free form and the salt form are saved as different structures and are assigned different MolPort IDs. As we process more and more supplier catalogs with the new process, you will notice that some of the previously available free-form compounds become unavailable and new MolPort IDs become available (for the salt forms of the structures).
We are planning to add new fields to the MolPort database export files and API. We will post more details in the near future.



